Abstract
Background Clonal hematopoiesis of indeterminate potential (CHIP) increases with age and is a risk factor for blood cancer, cardiovascular disease (CVD) and other diseases. Recent experimental evidence suggests a causal link between CHIP and CVD, however it is not known whether individuals with CHIP are more likely to benefit from preventive interventions for CVD, such as low-dose aspirin. ASPREE was a double-blind, randomized, placebo-controlled trial comparing 100mg aspirin with placebo on disability-free survival (DFS, composed of death, dementia and physical disability) in initially healthy participants aged ≥70y (≥65y for US minorities of Black and Hispanic background). We measured CHIP in serial blood samples of 11,298 ASPREE participants at baseline and/or year 3 (Y3), and assessed incidence of CHIP and its association with various clinical outcomes. We also examined whether randomization to aspirin had a differential effect on clinical outcomes according to the presence of CHIP at study entry.
Methods CHIP was measured in 19 CHIP-associated genes using a custom targeted amplicon-based NGS assay, designed to have a sensitivity of 0.5-2% variant allele fraction (VAF). CHIP was defined as a VAF ≥ 2%. We evaluated the association of CHIP at baseline and Y3, defined as a categorical variable (VAF< 2%, VAF 2-10%, VAF >10%), with clinical outcomes, including DFS, all-cause mortality, cancer and major adverse cardiovascular events (MACE, composed of fatal coronary heart disease, non-fatal myocardial infarction and ischemic stroke) using Y3-landmark Cox proportional hazards models. Risk factors for incident CHIP at Y3 were identified using multivariable logistic regression. Treatment heterogeneity of aspirin was assessed in CHIP subgroups overall and by the most common genes.
Results CHIP was present in 3,345/9,926 (34%) participants at baseline and in 3,685/9,812 (39%) at Y3. Of the 5,612 participants without CHIP at baseline who had a Y3 sample, 1,195 (25%) developed CHIP by Y3 (incidence 7% per year). Risk factors for incident CHIP included cancer history (odds ratio [OR] (95% CI) 1.20 [1.02-1.41] P=0.031), and family history of blood cancer (OR 1.50 [1.16-1.93] P=0.002).
With regards to clinical outcomes, the presence of CHIP at >10% VAF at baseline (but not VAF 2-10%) was associated with increased all-cause mortality (adjusted [aHR] [95% CI] 1.53 [1.23-1.90] P<0.001). This increased risk appeared to be driven by increased cancer-related mortality (baseline aHR 1.62, [1.19-2.22] p=0.002) more than cardiovascular-related mortality (baseline aHR 1.34 [0.84-2.12] p=0.21).
CHIP at >10% at baseline and Y3 was also associated with increased risk of blood cancer (HR 2.48 [1.59-3.88] and 3.24 [1.98-5.30], both P<0.001) and other cancers at Y3 (HR 1.31 [1.02-1.68] P=0.033). After adjustment for potential confounders, this association remained significant for blood cancers (aHR 2.29 [1.44-3.62] p<0.001) but not non-blood cancers (aHR 1.21 [0.98 -1.48] P=0.07). There was no evidence of an association between CHIP at 2-10% and >10% with MACE (aHR 0.91 [0.74-1.11] P=0.34 and aHR 0.73 [0.50-1.07] P=0.11, respectively).
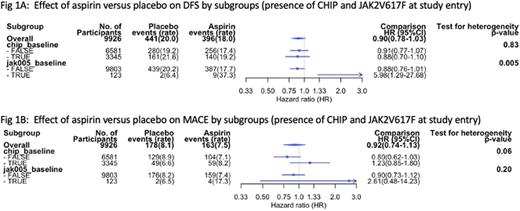
There was no evidence of a differential effect of aspirin on DFS according to presence of CHIP at study entry (CHIP HR 0.91 [0.77-1.07], no-CHIP HR 0.88 [0.70-1.10], test for heterogeneity P=0.83) or on MACE (CHIP HR 1.23 [0.85-1.80], no-CHIP HR 0.80 [0.62-1.03] heterogeneity P=0.06) - Figure 1. There was no subgroup difference for the effect of aspirin on incident cancer or cancer-related mortality.
Participants with detectable JAK2V617F (VAF > 0.5%, n=123) had worse outcomes if randomized to aspirin, including the primary outcome of DFS (Fig 1). There was no differential effect of aspirin in the JAK2V617F subgroup for MACE (Fig 1).
Conclusions This is the largest primary CVD prevention trial to evaluate the intervention effect according to presence of CHIP in healthy elderly. We found CHIP at >10% VAF was associated with increased all-cause mortality, which appeared to be driven largely by increased cancer-related mortality. There was no evidence that participants with CHIP were more likely to benefit from aspirin, including for MACE. Furthermore, aspirin appeared to reduce DFS for individuals with JAK2V617F mutation, although sub-group numbers were small. Our results suggest aspirin should not be used for primary prevention in older individuals with CHIP, including JAK2V617F.
Disclosures
McQuilten:Amgen: Research Funding; Abbvie: Research Funding; Beigene: Research Funding; CSL: Research Funding; BMS/Celgene: Research Funding; GSK: Research Funding; Janssen: Research Funding; Novartis: Research Funding; Sanofi: Research Funding; Takeda: Research Funding. Wong:Pacific Edge Limited: Consultancy, Current equity holder in publicly-traded company. Bick:TenSixteen Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Wood:Janssen-Cilag: Research Funding; Bristol-Myers Squibb: Research Funding; Abbvie: Research Funding; Sanofi: Research Funding; CSL Behring: Research Funding; Gilead: Research Funding; Beigene: Research Funding; Astra Zeneca: Research Funding; Roche: Research Funding; Novartis: Research Funding; Antengene: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal